Difference Between Organic Arsenic and Inorganic Arsenic
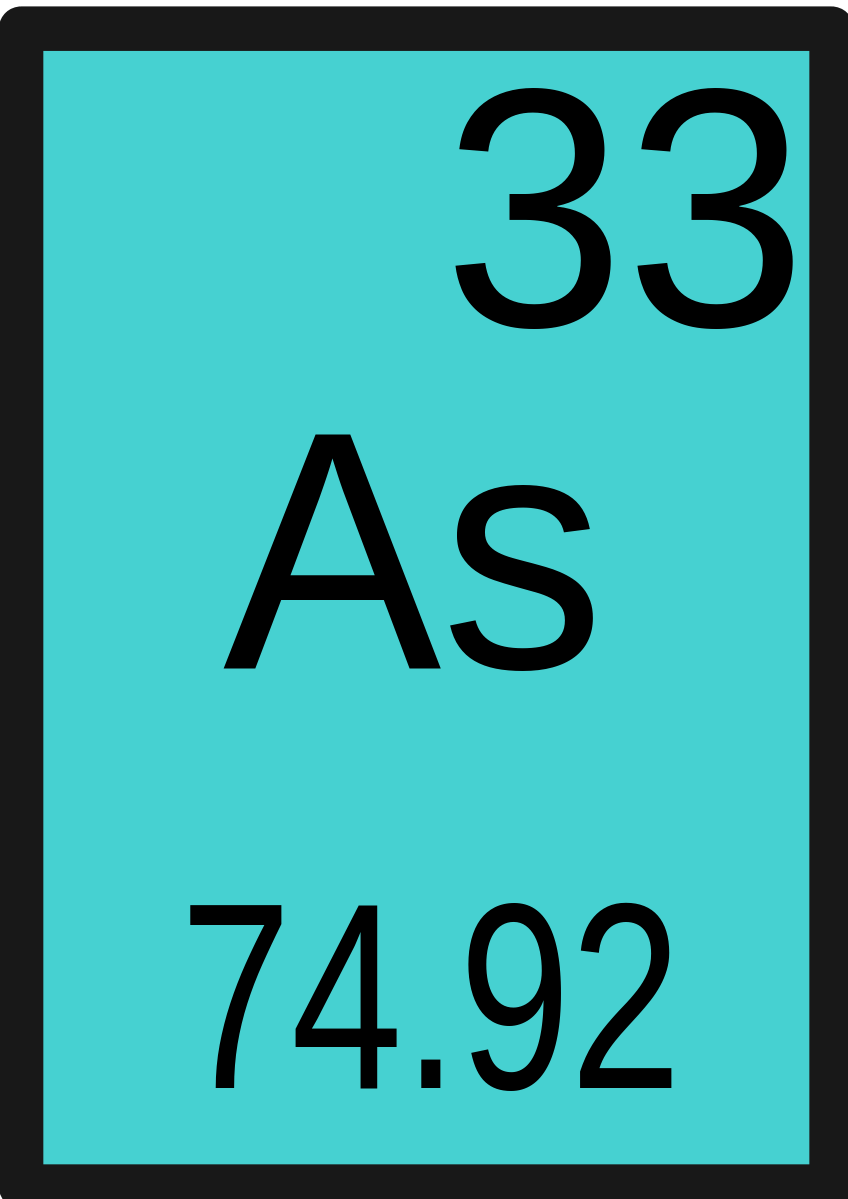
Arsenic is a chemical element, from group VA of the periodic table. Its physical and chemical properties intermediate between a non-metal and metal, so it is classified as a metalloid. Its relative atomic mass is 74.92. Arsenic can exist in different oxidation states: –3, 0, +3, and +5. It occurs in the form of three allotropic forms: yellow, black, and gray.
Arsenic is found in various organic and inorganic forms that enter the environment from natural sources and human activity. Water, soils, and many living organisms contain arsenic in low concentrations. A minimal amount of arsenic is beneficial for multicellular organisms. Daily ingestion above the required dose results in chronic arsenic intoxication, which can lead to Alzheimer’s, diabetes, cancer, etc. The arsenic intoxication has the so-called “embalming” effect and can preserve a corpse from decomposition for years.
The arsenic is known as a toxic element, but its toxicity depends on the molecular form and oxidation state, with the organic arsenic less toxic than the inorganic one.
What is Organic Arsenic?
Arsenic, occurring in plants and animals as a part of organic substances is known as organic arsenic.
Common organic arsenic compounds include:
- Arsanilic acid;
- Arsenobetaine;
- Cacodylic acid;
- Methylarsonic acid, etc.
The main source of arsenic intake for the people is the seafood. Shellfish, crustaceans, and seaweed, are known to have naturally high organic arsenic levels.
The toxicity of organic arsenic is low. In mice, no signs of toxicity are observed after an oral dose of 10 g/kg arsenobetaine. In humans, the organic arsenic is absorbed from the digestive system and excreted within a few days with insignificant changes in the chemical structure of the compounds, and with no harm to the organism.

What is Inorganic Arsenic?
Arsenic occurs in nature mostly in inorganic substances, containing oxygen, chlorine, and sulfur.
Common inorganic arsenic compounds include:
- Arsenic acid;
- Arsenic trioxide;
- Arsenic trichloride;
- Arsenic pentoxide;
- Calcium arsenate;
- Lead arsenate;
- Sodium arsenite, etc.
Inorganic arsenic occurs in water, soils, and some terrestrial foods, like rice. It has been estimated that in different parts of the world between 25 and 100% of the arsenic in terrestrial foods is inorganic.
The toxicity of inorganic arsenic is high. For arsenic trioxide, the LD50 (50% lethal dose) in rats is 20 mg/kg.
Inorganic arsenic is very toxic to humans. It can cause cancer of the lungs, skin, bladder, liver, kidney, etc., may damage the stomach, intestines, nerves, skin, and other tissues. Direct skin contact may damage the skin and cause swelling and redness. Low levels of exposure may result in:
- Abnormal heart rhythm;
- Blood vessel damage;
- Decreased production of red and white blood cells;
- Nausea, vomiting, and diarrhea;
- “Pins and needles” in feet and hands.
Difference Between Organic Arsenic and Inorganic Arsenic
Definition
Organic Arsenic: Arsenic, occurring in plants and animals as a part of organic substances is known as organic arsenic.
Inorganic Arsenic: Arsenic, occurring in nature in inorganic substances is known as inorganic arsenic.
Examples
Organic Arsenic: Common organic arsenic compounds include arsanilic acid, arsenobetaine, cacodylic acid, methylarsonic acid, etc.
Inorganic Arsenic: Common inorganic arsenic compounds include arsenic acid, arsenic trioxide, arsenic trichloride, arsenic pentoxide, calcium arsenate, lead arsenate, sodium arsenite, etc.
Occurrence
Organic Arsenic: The main source of arsenic intake for the people is the seafood. Shellfish, crustaceans, and seaweed, are known to have naturally high organic arsenic levels.
Inorganic Arsenic: Inorganic arsenic occurs in water, soils, and some terrestrial foods, like rice. Between 25 and 100% of the arsenic in terrestrial foods is inorganic.
Toxicity
Organic Arsenic: The toxicity of organic arsenic is low. In mice, no signs of toxicity are observed after an oral dose of 10 g/kg arsenobetaine. In humans, the organic arsenic is excreted within a few days with no harm to the organism.
Inorganic Arsenic: The toxicity of inorganic arsenic is high. For arsenic trioxide, the LD50 in rats is 20 mg/kg.
Adverse effects
Organic Arsenic: The toxicity of organic arsenic is low and it does not cause adverse effects.
Inorganic arsenic: Inorganic arsenic can cause cancer of the lungs, skin, bladder, liver, kidney, damage of the stomach, intestines, nerves, skin, etc. Bigger doses can be lethal.
Organic Vs. Inorganic Arsenic: Comparison Chart

Summary:
- Arsenic is a metalloid from group VA of the periodic table.
- Water, soils, and many living organisms contain arsenic in low concentrations.
- Arsenic, occurring in plants and animals as a part of organic substances is known as organic arsenic.
- Arsenic, occurring in nature in inorganic substances is known as organic arsenic.
- Common organic arsenic compounds include arsanilic acid, arsenobetaine, cacodylic acid, methylarsonic acid, etc. Common inorganic arsenic compounds include arsenic acid, arsenic trioxide, arsenic trichloride, arsenic pentoxide, calcium arsenate, lead arsenate, sodium arsenite, etc.
- The main source of arsenic intake for the people is the seafood. Shellfish, crustaceans, and seaweed, are known to have naturally high organic arsenic levels. Inorganic arsenic occurs in water, soils, and some terrestrial foods.
- The arsenic is known as a toxic element, but its toxicity depends on the molecular form and oxidation state, with the organic arsenic less toxic than the inorganic one.
- The toxicity of organic arsenic is low. In mice, no signs of toxicity are observed after an oral dose of 10 g/kg arsenobetaine. In humans, the organic arsenic is excreted within a few days with no harm to the organism. The toxicity of inorganic arsenic is high. For arsenic trioxide, the LD50 in rats is 20 mg/kg.
- The organic arsenic is low and it does not cause adverse effects. Inorganic arsenic can cause cancer of the lungs, skin, bladder, liver, kidney, damage of the stomach, intestines, nerves, skin, etc. Bigger doses can be lethal.
- Difference Between Gallstones and Cholecystitis - September 5, 2021
- Difference Between Constipation and Cramping - August 4, 2021
- Difference Between Whole Genome Sequencing and Microarray - May 6, 2021
Search DifferenceBetween.net :
Leave a Response
References :
[0]Image credit: https://commons.wikimedia.org/wiki/File:Arsenic.svg
[1]Image credit: https://upload.wikimedia.org/wikipedia/commons/thumb/4/4d/Maitotoxin_2D_structure.svg/2000px-Maitotoxin_2D_structure.svg.png
[2]De, A. A Text Book of Inorganic Chemistry. New Delhi: New Age Publishers. 2003. Print.
[3]Housecroft, C., A. Sharpe. Inorganic Chemistry. London: Pearson Education Limited. 2012. Print.
[4]Hoffman, R. Organic Chemistry (2nd Edition). Hoboken: John Wiley & Sons, Inc. 2004. Print.
