Difference Between Acetone and MEK
Acetone and MEK are the most common organic solvents used for cleaning and thinning applications. Both are potent chemical solvents that share the ketone trait and have a sharp odor but no color. Because both can be used as solvent thinner and cleaners, many believe that they can be used interchangeably. But they are not the same. Before you decide which solvent is right for you, first you need to be aware of these Acetone and MEK facts.

Acetone
Acetone is an organic compound commonly used to make plastics and other industrial stuffs. Also known as dimethyl ketone or propanone, acetone may also be used in household products like cosmetics and personal care products. It is a chemical used to remove paint, lacquer, or varnish. It is mostly used in the formulation of nail polish removers. Acetone is a colorless liquid solvent which is often used as a precursor for other materials. It evaporates easily and is flammable. It is a common organic solvent used for thinning and cleaning.
Acetone is also used in formulating low-VOC and low-HAP products. It is found naturally in plants, trees, forest fires, and volcanic gases. It is also found in the human body as a byproduct of metabolism. It is a primary ingredient in most nail polish removers. It is the simplest ketone which was traditionally obtained from the destructive distillation of acetic acid and acetates. The correct formula was determined by Jean-Baptiste-André Dumas and Justus von Liebig in 1832. The name acetone has been used starting 1839.
MEK
Methyl Ethyl Ketone, or MEK is a fast evaporative liquid solvent found in printing inks, adhesives, and surface coatings. It is a colorless liquid solvent that is related to acetone and is highly volatile. It is sweet and has a shard acetone-like odor. MEK is commercially manufactured from n-butenes in a metal-catalyzed hydrogenation reaction through rapid formulation of 2-butanol. It is a commonly used ingredient in varnishes and glues. It is mostly used as a component of a mixture of organic solvents. It can be found in a wide variety of natural products.
MEK is a versatile industrial chemical widely used for cleaning and thinning polyester resins and gel coats. It is mostly used in industrial setting and in common household products, such as adhesives, ink paints, cleaning fluids, lubricating oil dewaxing, oil refining, and protective coatings. It is classified by the Environmental Protection Agency (EPA) as a highly toxic, ignitable hazardous substance. Acute inhalation exposure to MEK can cause irritation to the eyes, nose, throat, and skin. At high concentrations, it can have serious health impact.
Difference between Acetone and MEK
Water Solubility
– Both Acetone and MEK are water soluble organic compounds. But acetone has a low boiling point, which makes it easy to evaporate for concentration and solvent exchange. MEK, on the other hand, has a higher boiling point which makes it a more potent and powerful cleaning agent.
Toxicity
– Both acetone and MEK are low-VOC solvents meaning they are less toxic solvents and are generally safe to use when stored under pre-defined conditions. However, MEK is a highly volatile chemical substance that is commonly used in industrial applications and household products, such as paints, inks, adhesives, cleaning agents, dewaxing agents, and protective coatings. MEK is stronger than acetone and is classified by the Environmental Protection Agency (EPA) as a highly toxic, ignitable hazardous substance.
Evaporation Rate
– The evaporation rate is the rate at which a substance will vaporize compared to the vaporization rate of a reference substance under same conditions. MEK has a slower evaporation rate than acetone, which is why it is able to maintain a constant boil when mixed with water. Acetone has a relatively higher evaporation rate because it has the weakest intermolecular forces, so it evaporates more quickly.
Acetone vs. MEK: Comparison Chart
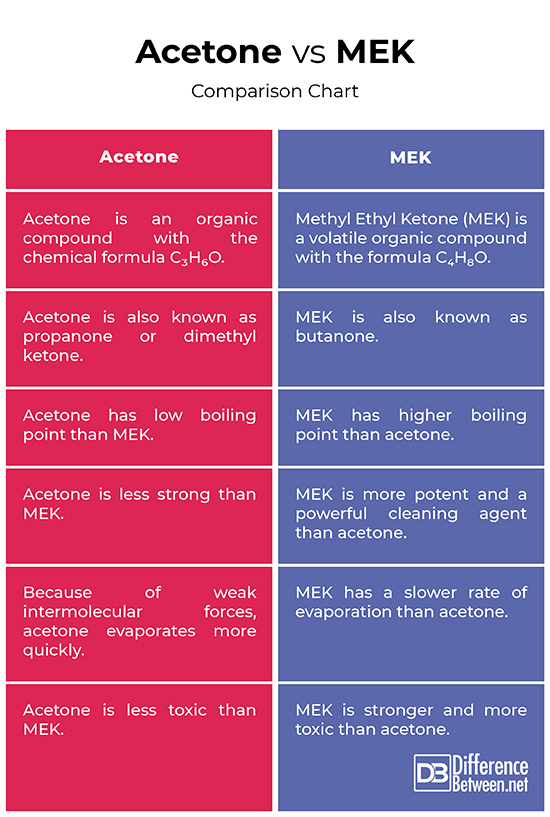
Summary of Acetone vs. MEK
Though a great alternative, MEK is often replaced with acetone in industrial applications because acetone is less toxic than MEK. Though classified by the Environmental Protection Agency (EPA) as highly toxic, no studies were to be found regarding human deaths following inhalation, oral, or dermal exposure to MEK. It is sweet and has a shard acetone-like pungent odor. Acetone is a colorless, flammable chemical compound with a higher rate of evaporation but lower boiling point. Acetone is less toxic than MEK but carries most of the same health and safety risks as MEK.
Is acetone safer than MEK?
Both share the ketone trait, but in terms of toxicity, both are low-VOC solvents with low levels of toxicity. However, MEK is a volatile solvent and is exceptionally stable. Acute inhalation exposure to MEK can cause irritation to the eyes, nose, throat, and skin.
Why is MEK banned?
While MEK has been classified by the Environmental Protection Agency (EPA) as highly toxic, it is not banned nationally, but there are some areas with strict VOC laws where MEK use is banned. However, it can be hazardous when not used properly.
What is MEK substitute used for?
MEK substitutes provide a safe and effective alternative to hazardous chemical substances. They are used as great industrial solvents for cleaning and thinning activities. Acetone is a chemical solvent used predominantly in paint removers, nail polish removers, and varnish removers.
Is MEK the same as paint thinner?
While both may be used for some of the same applications, including removing dried paint, varnish, and lacquer, MEK is not the same as paint thinner. Acetone has tons of other applications than just removing paint.
- Difference Between Caucus and Primary - June 18, 2024
- Difference Between PPO and POS - May 30, 2024
- Difference Between RFID and NFC - May 28, 2024
Search DifferenceBetween.net :
Leave a Response
References :
[0]Whim, B.P. and P.G. Johnson. Directory of Solvents. Berlin, Germany: Springer, 2012. Print
[1]Wypych, George. Handbook of Solvents. Ontario, Canada: ChemTec Publishing, 2001. Print
[2]Myers, Richard L. The 100 Most Important Chemical Compounds: A Reference Guide. California, United States: ABC-CLIO, 2007. Print
