Difference Between Nitrate and Nitrite
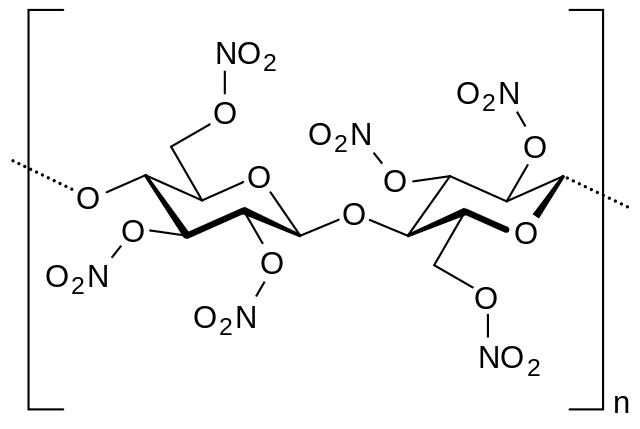
What is Nitrate?
The nitrate ion (NO3) is a conjugated base of the nitric acid. It consists of one nitrogen and three oxygen atoms. The nitrogen atom is located in the center and surrounded by oxygen atoms, which are identically bonded in a planar trigonal conformation. The molar mass of the nitrate anion is 62 g/mol. It dissociates in water to give nitrate hydroxyl ions.
Nitrates are chemical compounds, salts of the nitric acid. They are easily soluble. The nitrates are used in:
- Agriculture (mineral fertilizers);
- Food industry (colorants and preservatives);
- Production of paints, medicines, plastics, glass, explosives, etc.
Nitrates are found in soil, water, and food (of plant and animal origin). At low concentrations (1-40 mg/m3) they are also present in the air as. The nitrates are naturally synthesized by nitrogenous bacteria as an intermediate step in the formation of nitrogen. The natural concentrations in plants and water are generally low. Their quantity in arable land and in water normally does not exceed 10 mg/l. It can be increased by the use of nitrogen fertilizers, the introduction of livestock manure and other sources into the soil.
The nitrate content of the plants varies, depending on their quantity in the soil. From the soil solution, plants extract nitrogen, amino acids, proteins, vitamins and other substances, mainly in the form of nitrates. If the plant extracts more nitrates than the enzyme nitrate reductase can process, they accumulate in it. The activity of nitrate reductase depends on factors such as light, temperature and water stress.
Nitrates themselves are not toxic. Hazardous to the human health are the nitrites and nitrosamines. Both of them can form from the nitrates before or after ingestion of food or water.
Nitrates enter the human body via food and drinking water. Foods rich in nitrates are the vegetables and meat products (sausages, smoked meats). Much less to negligible is the nitrate content in dairy products and fish. Some plants have the ability to accumulate more nitrates. These are lettuce, carrots, spinach, dill, red beets, red radish, zucchini, broccoli, etc.
Nitrates in the air may act as respiratory irritants. Studies have shown an increase in asthmatic attacks associated with increased nitrate content in the air.
The maximum permissible 24 hour dose of potassium and sodium nitrate for humans is up to 5 mg/kg. Poisoning occurs at a dose of 4 g/24h, the human lethal dose is 8-15 g/24h.
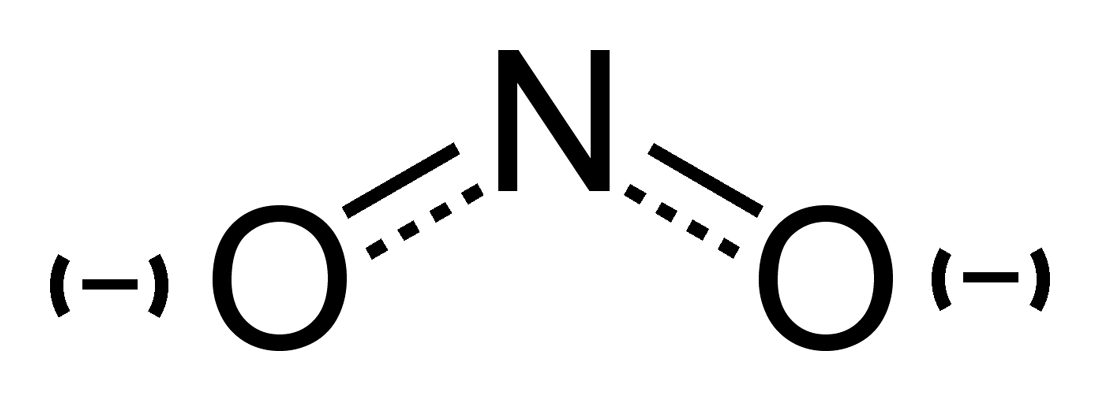
What is Nitrite?
The nitrite ion (NO2) is a conjugated base of the nitrous acid. The anion is symmetric. The nitrogen atom is located in the center and surrounded by two oxygen atoms, which are identically bonded. The molar mass of the nitrite anion is 46 g/mol.
Nitrites are chemical compounds, salts or esters of the nitrous acid. They are widely used in the production of meat and fish products. They have bactericidal action. Their reaction with myoglobin gives the meat a fresh look and pink-red color.
Humans can be exposed to nitrates in several ways. The excessive nitrogen fertilization increases the nitrate content in plants, and respectively in humans (via food). In the human body, the nitrates are reduced to nitrites. The higher plants can also assimilate nitrite from the soil. Microbiological conversion of nitrates into nitrites can occur when fresh vegetables are stored, especially at room temperature.
Nitrates, when ingested, are categorized as “probably carcinogenic to humans”. They bind to the hemoglobin in the blood and form a compound called methemoglobin. It is unable to carry oxygen to the organs and tissues, which leads to a condition known as methemoglobinemia, characterized by bruising of the skin and mucous membranes (cyanosis).
Nitrates are most dangerous when converted to nitrite before they are swallowed. This conversion can occur when the food is stored improperly (high temperature and reduced oxygen content in the room) or during cooking, especially during frying.
Nitrites damage the cellular structures of different organs and systems directly, depending on the dose. They disturb the transport of oxygen, cause toxic damage to enzyme systems, cause mutagenic, carcinogenic and other subcellular changes, reduce the immune system’s activity.
In low acidity of gastric acid, nitrites can be converted to nitrosamines, which have a carcinogenic effect.
The maximum allowable dose of nitrite for 24 hours is 0.2 mg/kg. The human lethal dose is 0.18-2.5 g/24 h. It is believed that taking 0.5 g of nitrite can lead to mild, and 1-2 g – to severe poisoning.
Difference Between Nitrate and Nitrite
-
Definition
Nitrate: The nitrate ion (NO3) is a conjugated base of the nitric acid. Nitrates are chemical compounds, salts of the nitric acid.
Nitrite: The nitrite ion (NO2) is a conjugated base of the nitrous acid. Nitrites are chemical compounds, salts or esters of the nitrous acid.
-
Structure
Nitrate: The nitrate ion consists of one nitrogen and three oxygen atoms. The nitrogen atom is located in the center and surrounded by oxygen atoms, which are identically bonded in a planar trigonal conformation.
Nitrite: The nitrite ion consists of one nitrogen and two oxygen atoms. The nitrogen atom is located in the center and surrounded by the oxygen atoms, which are identically bonded.
-
Molar mass
Nitrate: The molar mass of the nitrate anion is 62 g/mol.
Nitrite: The molar mass of the nitrite anion is 46 g/mol.
-
Use
Nitrate: The nitrates are used in the agriculture (mineral fertilizers), food industry (colorants and preservatives), production of paints, medicines, plastics, glass, explosives, etc.
Nitrite: The nitrites are used in the production of meat and fish products.
-
Hazards
Nitrate: Nitrates in the air may act as respiratory irritants. Nitrates themselves are not toxic when swallowed. Hazardous to the human health are the nitrites and nitrosamines, which can form from the nitrates.
Nitrite: Nitrates are categorized as “probably carcinogenic to humans”. Depending on the dose they can damage the cellular structures of different organs and systems, disturb the transport of oxygen, cause toxic damage to enzyme systems, cause mutagenic, carcinogenic and other subcellular changes, reduce the immune system’s activity.
-
Hazardous concentrations
Nitrate: The maximum permissible 24 hour dose of potassium and sodium nitrate for humans is up to 5 mg/kg. Poisoning occurs at a dose of 4 g/24h, the human lethal dose is 8-15 g/24h.
Nitrite: The maximum allowable dose of nitrite for 24 hours is 0.2 mg/kg. Poisoning occurs at a dose of 0.5 g/24 h, the human lethal dose is 0.18-2.5 g/24 h.
-
Examples
Nitrate: Potassium nitrate, sodium nitrate.
Nitrite: Sodium nitrite, ammonium nitrite.

Summary:
- The nitrate ion (NO3) is a conjugated base of the nitric acid. Nitrates are chemical compounds, salts of the nitric acid.
- The nitrite ion (NO2) is a conjugated base of the nitrous acid. Nitrites are chemical compounds, salts or esters of the nitrous acid.
- The nitrate ion consists of one nitrogen and three oxygen atoms. The nitrogen atom is located in the center and surrounded by oxygen atoms, which are identically bonded in a planar trigonal conformation. The nitrite ion consists of one nitrogen and two oxygen atoms. The nitrogen atom is located in the center and surrounded by the oxygen atoms, which are identically bonded.
- The molar mass of the nitrate anion is 62 g/mol, while the molar mass of the nitrite anion is 46 g/mol.
- The nitrates are used in the agriculture, food industry, production of paints, medicines, plastics, glass, explosives, etc. The nitrites are used in the production of meat and fish products.
- Nitrates in the air may act as respiratory irritants. Nitrates themselves are not toxic when swallowed. Hazardous to the human health are the nitrites and nitrosamines, which can form from the nitrates. Nitrates are categorized as “probably carcinogenic to humans”. Depending on the dose they can damage the cellular structures of different organs and systems, disturb the transport of oxygen, cause toxic damage to enzyme systems, cause mutagenic, carcinogenic and other subcellular changes, reduce the immune system’s activity.
- The maximum permissible 24 hour dose of potassium and sodium nitrate for humans is up to 5 mg/kg. Poisoning occurs at a dose of 4 g/24h, the lethal dose is 8-15 g/24h. The maximum allowable dose of nitrite for 24 hours is 0.2 mg/kg. Poisoning occurs at a dose of 0.5 g/24 h, the lethal dose is 0.18-2.5 g/24 h.
- Difference Between Gallstones and Cholecystitis - September 5, 2021
- Difference Between Constipation and Cramping - August 4, 2021
- Difference Between Whole Genome Sequencing and Microarray - May 6, 2021
Search DifferenceBetween.net :
Leave a Response
References :
[0]Image credit: https://upload.wikimedia.org/wikipedia/commons/b/b0/Nitrite-ion-resonance-hybrid.png
[1]Image credit: https://upload.wikimedia.org/wikipedia/commons/thumb/4/49/Cellulose_nitrate.svg/640px-Cellulose_nitrate.svg.png
[2]De, A. A Text Book of Inorganic Chemistry. New Delhi: New Age Publishers. 2003. Print.
[3]Housecroft, C., A. Sharpe. Inorganic Chemistry. London: Pearson Education Limited. 2012. Print.
[4]Lazarov, D. Inorganic Chemistry. Sofia: St. Kliment Ohridski. 1995. Print.
