Difference Between Nitrification And Denitrification
Nitrification
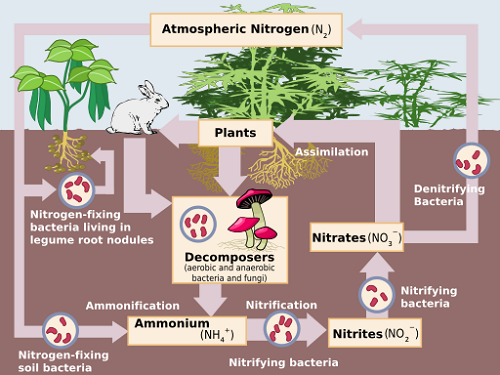
Nitrification is the biological transformation of ammonium (NH4+) to nitrate (NO3–) by oxidation. Oxidation is defined as the loss of electrons by an atom or compound, or an increase in its oxidation state. The process is facilitated by two types of nitrifying aerobic bacteria that require the presence of oxygen molecules dissolved in their surroundings, to survive. [i]
First, chemoautrophic bacteria (mainly those of the genus Nitrosomonas) convert ammonia (NH3) and ammonium to nitrite (NO2–). “Chemoautrophic” refers to the bacteria’s ability to create its own nutrients from an inorganic source, namely CO2. The process is represented by the chemical equation:
2NH4+ + 3O2 → 2NO2– + 2H2O + 4H+ + energy
Then bacteria primarily from the Nitrobacter group convert nitrite to nitrate in the following reaction:
2NO2– + O2 → 2NO3– + energy
These reactions take place simultaneously and quite rapidly – usually within days or weeks. It is important that nitrite is completely converted to nitrate in soils, since nitrite is toxic to plant life.
Nitrates present in the soil are the main source of nitrogen used by plants. [ii] Thus the transition of nitrogen from one form to another, known as the nitrogen cycle, is an important part of the agricultural industry.[iii]
Before these steps take place, organic nitrogen is broken down by heterotrophic bacteria by hydrolysis to form ammonium and ammonia in a process known as ammonification. i Ammonia may be found in urea from animal wastes, composts and decomposing cover crops or crop residues. Ammonium is found in most fertilizers.
Nitrifying bacteria are more sensitive to environmental stresses than other types of soil bacteria. When soil has been saturated with moisture for prolonged periods, soil pores fill with water, limiting oxygen supply. Nitrifying bacteria require aerobic conditions to function, thus flooding restricts nitrification.
Dry soils tend to have high salt concentration and the resulting salinity negatively impacts the bacteria’s nitrifying activity. This is because increased osmolarity raises the amount of energy required by microorganisms to move water across their cell membranes. Water is also essential for movement of solutes, such as nitrates, through the soil. ii
Nitrifying bacteria perform best at a pH between 6.5 and 8.5 and temperatures between 16 and 35 degrees C. i Nitrification rates are slower in very acidic soils, while high alkalinity reduces Nitrobacter activity, causing an unfavourable build-up of nitrite in the soil.
The soil pH may also be affected by the particular source of ammonium nitrified. For example, monoammonium phosphate (MAP) solution is much more acidic than diammonium phosphate (DAP); thus use of DAP results in higher nitrification rates than MAP.
The majority of the bacteria are found in the upper surface layer, thus nitrification declines when tillage practices are not managed properly.
Soils with high clay content have larger particles and more micropore space for bacterial growth, as well as greater retention of ammonium due to higher cation exchange capacity. ii Water relations and soil physical properties may be improved by reduced-till cultivation.
Nitrification may be inhibited by the presence of heavy metals and toxic compounds, or excessively high concentrations of ammonia.
Sometimes it may be beneficial to keep nitrogen in the soil in the form of ammonium. This prevents nitrogen loss (by leaching of nitrates) and nitrogen gas escape (through denitrification). Nitrification inhibitors used commercially include dicyandiamide and nitrapyrin.
Denitrification
Denitrification is the biological transformation of nitrate to nitrogenous gases by reduction. It always follows nitrification i and the reaction sequence may be represented as follows:
NO3– → NO2– → NO → N2O → N2[iv]
The process is facilitated by facultative bacteria; these are bacteria that do not require the presence of free oxygen for respiration. Denitrifying bacteria are heterotrophic organisms as they need an organic food source, in the form of carbon, to survive. Denitrification may start as rapidly as minutes after stimulation of the process.
Denitrification may be detrimental to crop production, since nitrogen, a nutrient essential for plant growth, is lost to the atmosphere during the process. However, it is beneficial to aquatic habitats and in industrial or sewage wastewater treatment, as nitrate concentration in the water is lowered. i
Leaching or runoff from crops due to fertilizer treatments may cause excess quantities of this nutrient to end up in water bodies, where nitrogenous compounds have various harmful effects on both human and aquatic life. iv
Ammonia is toxic to fish species and stimulates algae growth, reducing oxygen levels in water and resulting in eutrophication. Nitrates cause liver damage, cancers and methemoglobinemia (oxygen deficiency in infants), while nitrites react with organic compounds called amines to form carcinogenic nitrosamines. ii
When oxygen levels in soils or water are depleted (anoxic conditions), denitrifying bacteria break down nitrates for use as an oxygen source. This commonly occurs in waterlogged soils where oxygen levels are low. Nitrate is reduced to nitrous oxide (N2O) and once more to nitrogenous gas. These gas bubbles escape into the atmosphere. i
The gas formed by denitrifiers depends on conditions in the soil or water and what kind of microbial community is present. Less oxygen tends to result in more nitrogen gas being formed, the most common product of denitrification. Nitrogen gas forms the main component of air. The second most common product formed is nitrous oxide, a greenhouse gas that also erodes the Earth’s ozone layer. iv
Denitrifying bacteria are less sensitive to toxic chemicals than nitrifiers and function optimally at a pH between 7.0 and 8.5 and warmer temperatures between 26 and 38 degrees C. Denitrification occurs mostly in the topsoil, where microbial activity is highest.
Denitrifiers require sufficient nitrate concentration and a soluble carbon source; the highest rates occur when using methanol or acetic acid. Organic carbon may be found in manure, compost, cover crops and crop residues. i
Minimizing denitrification in crop soils is achieved by maintaining the minimum concentration of nitrate necessary for plant growth, such as use of controlled-release fertilizers. Another method is inhibiting nitrification, which reduces the levels of nitrate available for denitrification.
Denitrification levels range widely across a single field, due to many factors such as soil properties (including aggregation, macropores and wetness) and variations in fertilizer, organic matter and crop residue distribution.
Nitrogen fertilizer types, as well as application methods, have been reported to affect denitrification. For example, coated controlled-release fertilizers, as well as fertigation and broadcast applications, cause lower nitrous oxide emissions than dry granular urea and concentrated band applications. Deeper placement of nitrogen also decrease these emissions.
Dry periods followed by a sudden rainstorm are often a trigger for denitrification, which can be managed with drainage systems and subsurface drip irrigation. iv
Summary
Nitrification
- Follows ammonification process
- Transformation of ammonium to nitrate
- Oxidation reaction
- Facilitated by two main types of chemoautrophic aerobic bacteria: Nitrosomonas and Nitrobacter
- Two –step process: conversion of ammonium to nitrite, then conversion of nitrite to nitrate
- Creates a nitrogen nutrient form available for absorption by plant roots
- Reactant (ammonium) found in urea from animal wastes and fertilizers, composts and decomposing cover crops or crop residues
- Nitrifiers more sensitive to environmental stresses
- Inhibited by flooding, high salinity, high acidity, high alkalinity, excessive tilling and toxic compounds
- Favoured by aerobic conditions, pH between 6.5 and 8.5, temperatures between 16 and 35 degrees C and high clay content
Denitrification
- Follows nitrification process
- Transformation of nitrate to nitrogenous gases, mainly nitrogen and nitrous oxide
- Reduction reaction
- Facilitated by heterotrophic facultative bacteria
- Sequence of steps: conversion of nitrate to nitrite, to nitric oxide, to nitrous oxide and finally to nitrogen
- Decontaminates wastewater and aquatic systems by lowering nitrate levels
- Reactant (nitrate) formed by nitrification, while carbon sources for denitrifiers are found in manure, cover crops and crop residues, or provided by methanol or acetic acid
- Denitrifiers less sensitive to environmental stresses
- Inhibited by reduced nitrification, lowered nitrate levels, deep placement of coated controlled-release fertilizer and soil drainage
Favoured by flooding, anoxic conditions, pH between 7.0 and 8.5, temperatures between 26 and 38 degrees C, sufficient supply of nitrates and soluble carbon and concentrated band applications of dry granular urea.
- Difference Between Hybrid And GM Seeds - February 10, 2017
- The Differences between Plants and Protists - February 7, 2017
- Difference Between Schist And Gneiss - February 3, 2017
Search DifferenceBetween.net :
1 Comment
Leave a Response
References :
[0][i] Nitrification & Denitrification. Unpublished scientific resource. The Water Planet Company, Connecticut. [Online]. PDF available: kisi.deu.edu.tr/orhan.gunduz/turkce/dersler/Nitrification_and_Denitrification.pdf [2017, January 16].
[1][ii] Nitrification. Unpublished bulletin series (Nitrogen Notes Number 4). International Plant Nutrition Institute, Georgia. [Online]. PDF available: http://www.ipni.net/publication/nitrogen-en.nsf/book/7F7F448C4D064A5985257C13004C83A3/$FILE/NitrogenNotes-EN-04.pdf [2017, January 16].
[2][iii] Chang NB. 2011. Making a Progress to Speed up the Nitrification and Denitrification Processes in Novel Biosorption Activated Media: Can Archaea be in Concert with Anammox? J Bioprocess Biotechniq, 1:2
[3][iv] Denitrification. Unpublished bulletin series (Nitrogen Notes Number 5). International Plant Nutrition Institute, Georgia. [Online]. PDF available: http://www.ipni.net/publication/nitrogen-en.nsf/0/668099AE825517CB85257DD600054B8C/$FILE/NitrogenNotes-EN-5.pdf [2017, January 16].
[4]https://commons.wikimedia.org/wiki/File:Nitrogen_Cycle.svg
[5]https://commons.wikimedia.org/wiki/File:Nitrogen_Cycle.svg

I need 3 differences between nitrification and denitrification. Thank you.