Difference between hydrogen bonds and covalent bonds
The topic makes it very clear that the article is based on some concepts from chemistry. For those of you who know the basic concepts of chemical bonding, it is easy to comprehend that the discussion is about two types of bonds. As for others, let us just say that among the many chemical bonds that occur between atoms and molecules, we are going to discuss and differentiate two very important type of bonds, namely hydrogen bonds and covalent bonds.
It is very often for people to confuse the two. This is because of a vague definition offered to explain these relative to other type of bonds. The simplest definition that is offered is that a bond between two non-metals is usually covalent whereas a bond between a metal and a non-metal is ionic. These definitions are quite generalized and there are lot of exceptions as well as contradictions to it. First of all, it must be noted that all bonds between two non-metals do not fall under the category of covalent bonds; there are also other bonds, one of which is a hydrogen bond.
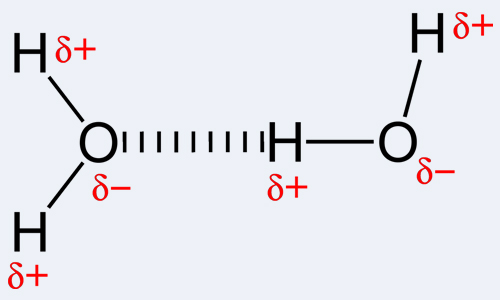
By definition, a covalent bond is a form of a chemical bond that occurs due to the sharing of electron pairs between the same or distinct atoms. Covalent bonding, in turn, refers to the stable balance of force (both attractive and repulsive) between atoms when they share electrons. The sharing allows each atom involved to attain an outer shell that is equivalent to a full valence shell or outer shell. This accounts for a stable configuration of electrons. In contrast to this, a hydrogen bond is actually the name of the electrostatic attraction between special types of molecules, known as polar molecules. The bond uniquely occurs when a hydrogen atom that is already bonded to a highly electronegative atom (one of the three; oxygen, nitrogen or fluorine) experiences another force of attraction from a nearby atom that is also highly electronegative. Note that hydrogen must be there for a hydrogen bond to occur, and hence the name of the bond. Also, one of the three above mentioned atoms should be bonded to it. This is because nitrogen, fluorine and oxygen are very electronegative, that is, attract electrons towards themselves. This makes the hydrogen behave as a positively charged particle as the negatively charged electrons have been attracted towards the corresponding nitrogen, fluorine or oxygen atom. Therefore, this hydrogen particle, that is now positive, is easily attracted towards another electronegative atom due to its negativity. To use the name hydrogen bond for this chemical interaction is more like using a misnomer because there is no true bond that is formed. In effect, there are di-pole to di-pole attractions.
The interactions that take place in covalent bonding include metal to metal bonding, three center two electron bonds, agostic interactions, π-bonding and σ-bonding. It is indeed notable that covalency is the greatest between atoms that have similar electronegativities. This implies that the two atoms need not be of the same element but should have electronegativity that is comparable and close to allow for stronger bonds. As opposed to this, hydrogen bonds are intermolecular, that is, occur between molecules or between different parts of one molecule. The hydrogen bonds are pretty strong; stronger than van der Waals forces but are weaker than covalent and ionic bonds. Examples of molecules where hydrogen bonding occurs includes water as well as some organic moleculses such as proteins, DNA etc.
Summary of differences expressed in points
1. Covalent bond-a chemical bond that occurs due to the sharing of electron pairs between same or distinct atoms, covalent bonding refers to the stable balance of force (both attractive and repulsive) between atoms when they share electrons, sharing allows each atom involved to attain an outer shell that is equivalent to a full valence shell or outer shell; a hydrogen bond is the electrostatic attraction between special types of molecules, known as polar molecules. The bond uniquely occurs when a hydrogen atom already bonded to a highly electronegative atom (one of the three; oxygen, nitrogen or fluorine) experiences another force of attraction from a nearby atom that is also highly electronegative
2. Covalent bonding can occur between a large variety of atoms; hydrogen bonds require hydrogen and one of oxygen, nitrogen or fluorine
3. Covalent bond are stronger than hydrogen bonds
- The difference between Royal icing and Buttercream icing - March 22, 2015
- Difference between stuffed and deep dish pizza - March 21, 2015
- Difference between Crane and Heron - March 20, 2015
Search DifferenceBetween.net :
2 Comments
Leave a Response
References :
[0]http://upload.wikimedia.org/wikipedia/commons/b/b5/Hydrogen-bonding-in-water-2D.png

Difference between hydrogen bonds and covalent bonds? I dont get it haha
So confusing..am going crazy