Difference Between Galvanic Cells and Electrolytic Cells
There are two types of electrochemical cells: galvanic cells – with spontaneous redox processes that allow continuous flow of electrons through the conductor, whereby the chemical energy is transformed into an electrical one; and electrolytic, where the redox reactions are influenced by an external source of current, where the electricity is converted in chemical energy.
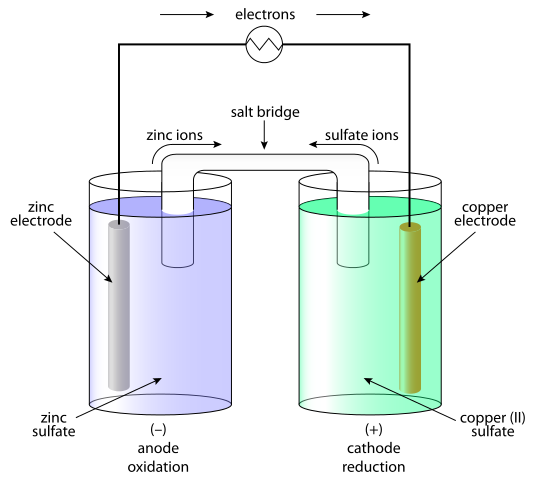
What is Galvanic Cell?
Galvanic cells are systems in which the chemical energy is transformed into electrical and as a result current is generated. In galvanic cells, direct current is generated as a result of the redox (oxidation-reduction) process. The galvanic element consists of two half-cells. The half-cell consist of the electrolyte and the immersed electrode in it. Between these half-cells a contact must be provided, connecting the electrolyte with a salt bridge or semi-conductive membrane and connecting the electrode with conductor. The separation of the redox process is explained by the electrodes’ behavior in relation to the electrolyte. The simplest option is that the half-cell is formed of a metal electrode immersed in an electrolyte containing ions correspondent with the electrode. The behavior of metals in the electrolyte depends on the reactivity of the metal i.e. its tendency to dissolve.

What is Electrolytic Cell?
The electrical current through the electrochemical cell can be initiated in two ways. The first is to connect electrodes with a conductor into a closed electric circuit. By closing the electric circuit it is possible to spontaneously induce electrode reactions on both phases of the metal/electrolyte. In addition, the energy of the current is released at the expense of the energy of a spontaneous chemical reaction. A cell that works this way is called a galvanic cell. This was explained above. Another way is to close the electric circuit by serial bonding of an external source of current as opposed to the voltage of the cell, wherein the external voltage is greater than the electromotive force of the cell. It drives the current in opposite direction from the direction of its spontaneous flow through the cell. Because of this, electrodes’ reactions in the cell have to be contrary to the direction of their spontaneous flow. Forced processes in an electrochemical cell under the influence of an external source of electrical current are called electrolysis, and the electrochemical cell in such a mode of operation is called an electrolytic cell.
Difference Between Galvanic and Electrolytic Cell
-
Definition of Galvanic and Electrolytic Cell
In galvanic cells there are spontaneous redox processes that allow continuous flow of electrons through the conductor, whereby the chemical energy is converted into electric. In an electrolytic cell, redox reactions take place under the influence of an external source, where the electricity is converted into a chemical energy. The redox reactions are non-spontaneous.
-
Technique of Galvanic and Electrolytic Cell
Galvanic cells generate electricity with the aid of chemical reactions. In electrolytic cells, an electric current is used for development of a chemical reaction, utilizing an external source along the way.
-
Design of Galvanic and Electrolytic Cell
Galvanic cells consists of two different electrodes immersed in solutions of their ions that are separated by a semipermeable membrane or a salt bridge. Electrolytic cells consist of an electrolytic container in which two electrodes are connected to a DC source. The electrolyte may be a melt or an aqueous solution of some salt, acid or alkali.
-
Electrode polarity in Galvanic and Electrolytic Cell
In galvanic cells the anode is the negative and the cathode is the positive electrode. In electrolytic cells, the opposite occurs.
-
Chemical reaction in Galvanic and Electrolytic Cell
In case of galvanic cell, the oxidation reaction takes place at the anode (negative electrode) where there is a surplus of negative charge. At the cathode, the reduction reaction happens, inducing a positive buildup of charge. In case of electrolytic cell an outside source is used to trigger a reaction. At the negative electrode, the electrons are pushed out of it – so the reduction phase will happen on the negative electrode. On the positive electrode the oxidation phase takes place – and this is the anode.
-
Application of Galvanic and Electrolytic Cell
Galvanic cells are used as a source of electrical current, and are more commonly referred to as batteries or accumulators. Electrolytic cells have different practical uses, some of them being making hydrogen and oxygen gas for commercial and industrial applications, electroplating, extracting pure metals from alloys and so on.
Galvanic vs. Electrolytic Cell: Comparison in tabular form

Summary of Galvanic vs. Electrolytic Cell
- An electrochemical cell is composed of two half-cells or electrodes whose contact is made via an electrolyte (ionic conductor). Half-cells, if separated, can be joined by a salt bridge (concentrated solution of electrolytes in agar-agar gel). The galvanic cell produces electric current based on a chemical change that occurs spontaneously in it. The electrolytic cell does exactly the opposite: the current results in a chemical change. In order for the cell to be galvanic, spontaneous chemical change must occur in it.
- Difference Between Thermodynamics and Kinetics - June 24, 2018
- Difference Between Welding and Soldering - June 24, 2018
- Difference Between Additive Colors and Subtractive Colors - June 20, 2018
Search DifferenceBetween.net :
1 Comment
Leave a Response
References :
[0]Petrucci, et al. “General Chemistry: Principles & Modern Applications”, 9th ed., NJ: Pearson/Prentice Hall, 2007.
[1]Bagotsky, V. S. “Fundamentals of Electrochemistry”, 2nd ed., NY: John Wiley & Sons, Inc., 2005.
[2]Zoski, S. “Handbook of Electrochemistry”, Netherlands: Elsevier Science, 2006.
[3]Image credit: https://upload.wikimedia.org/wikipedia/commons/thumb/a/a5/Galvanic_cell_labeled.svg/533px-Galvanic_cell_labeled.svg.png
[4]Image credit: https://upload.wikimedia.org/wikipedia/commons/thumb/3/3b/Electrochemical_element_with_salt_bridge.png/604px-Electrochemical_element_with_salt_bridge.png

Hi, On the the chart posted on the page that has the summary of electrolytic cell vs galvanic cell, if you take a look at the electrolytic cell in the chemical reaction block it says
“that the reduction will happen on the positive – anode” in parenthesis.
(should this say oxidation phase)?
However, the content within the blog states that
“In the case of electrolytic cell an outside source is used to trigger a reaction. At the negative electrode, the electrons are pushed out of it – so the reduction phase will happen on the negative electrode.”