What is Charcoal?
Charcoal is a carbon residue made from partially burned wood and other organic materials in which the burning took place with limited air supply.
History of charcoal production
Charcoal has been in use for thousands of years. The earliest use of charcoal is as a form of paint around 30,000 BC. Charcoal has also been important in metallurgy because charcoal burning can result in much higher temperatures than wood burning. The reason for this is that charcoal is dehydrated so that more of the energy goes into raising the temperature. This is useful for metallurgy since many metals melt only at very high temperatures. When wood burns, on the other hand, more energy goes into vaporizing the water present in the wood, taking away from the energy needed to raise the temperature. This results in lower temperatures for wood fires.

Formation of charcoal
Historically, charcoal was made by burning wood in a pit that was covered in soil or above ground and covered by a clay enclosure. The first evidence of charcoal production dates to about 3000 BC, when metal-working, namely with bronze and copper, became more common. The creation of charcoal requires a limited flow of air so that the burning wood smolders rather than bursting into flame. The limited air flow allows the burning of the wood to slow down. This results in the formation of charcoal.
Uses of charcoal
Today, charcoal has many uses. The most well-known uses of charcoal are metallurgy and cooking. Charcoal is also used for filtration of water and air because of its porous nature. The kind of charcoal that is most effective for metallurgy is charcoal that contains low amounts of Sulphur. This is because Sulphur usually ends up being transferred to whatever metal is being heated with the charcoal.

Activated Charcoal
Activated charcoal is created when ordinary charcoal is heated to a very high temperature. When this happens, the elements and compounds bound with the carbon atoms are removed and all the binding sites for carbon are “free” for binding with incoming molecules and atoms. This makes activated charcoal much more porous than ordinary charcoal, vastly increasing its surface area. In fact, a teaspoon of activated charcoal contains about the surface area of a football field because of its porosity.
The number of open binding sites for activated charcoal means that the substance is very good at removing contaminants. For this reason, activated charcoal has a number of medicinal and industrial uses, not all of which are scientifically proven.
Medical benefits of activated charcoal
Clinics and hospitals will often use activated charcoal as an antidote to ingested toxins, especially in the case of an emergency. Thus, if someone has ingested a poison or a toxin, ingesting activated charcoal can clear the system of that toxin. This, however, only works if it is done within 1-4 hours of consuming the toxin or poison and before the poison has been digested.
Activated charcoal has been recommended as treatment for many medical issues, including intestinal gas, kidney problems, skin diseases, and even teeth whitening. Theoretically, the activated charcoal will both clear toxins and gas that is causing gastrointestinal discomfort. It may be for this reason that activated charcoal is also recommended as an anti-flatulent. Activated charcoal has not been experimentally confirmed to be an effective treatment for many of these issues, but it does make sense scientifically. Furthermore, no serious negative side effects or risks have been attributed to activated charcoal.
Activated charcoal is useful as a filter, but it is possible for it to adsorb so many contaminants that there are no more binding sites or pores to take in incoming substances. As a result, activated charcoal can lose its effectiveness after repeated uses and fresh activated charcoal must be produced.
Similarities between Charcoal and Activated Charcoal
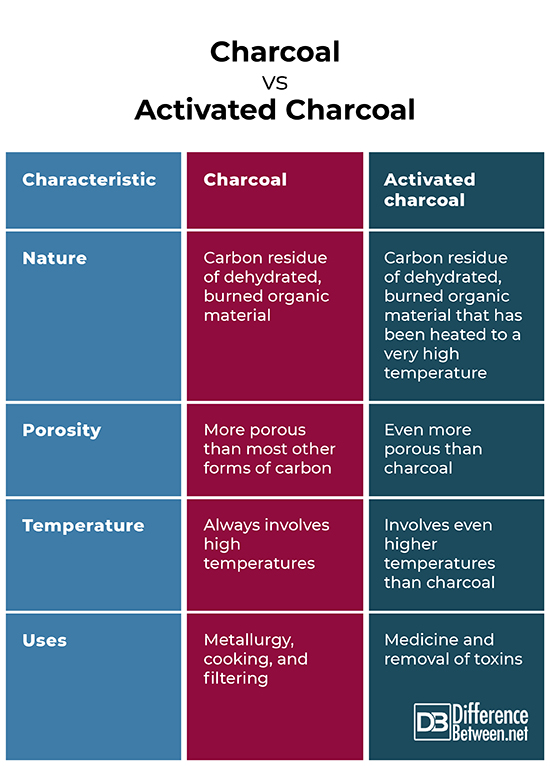
Charcoal and activated charcoal are both dehydrated residues left over from burning organic material. They are also both more porous than other forms of carbon which makes them useful for filtering material. Furthermore, they both are produced at high temperatures.
Differences between Charcoal and Activated Charcoal
There are many similarities between charcoal and activated charcoal, but they are not the same.
- Activated charcoal is produced at higher temperatures than charcoal.
- Activate charcoal is much more porous than charcoal.
- Activated charcoal is much more effective in filtering material and a more effective adsorbent than charcoal.
- Activated charcoal is more commonly used in medicine than charcoal.
Charcoal vs. Activated Charcoal
Summary of Charcoal vs. Activated Charcoal
Charcoal represents the dehydrated remains of burned organic material, usually wood. Charcoal has been produced for thousands of years by burning wood with limited air flow. This was first done in pits covered in soil or in kilns made of clay. Charcoal is useful for metallurgy because burning charcoal can produce much hotter fires than wood burning because more energy goes into raising the temperature. Charcoal’s primary uses are in cooking as well as metallurgy. Activated charcoal is made when charcoal is superheated and all the binding sites of the carbon making up the charcoal are freed, allowing incoming molecules and atoms to bind with them. This makes activated charcoal effective at adsorbing toxins in air and water. For this reason, activated charcoal has a variety of medical uses and is used as an antidote to many poisons, kidney problems, skin diseases, and is even used in teeth whitening. Activated charcoal is also used as a filter. Although it is good at removing toxins, it can lose its effectiveness over time as it adsorbs more material. As a result, activated charcoal does occasionally need to be replaced to maintain its effectiveness as a filter and contaminant removal agent.
- Difference Between Environmental Performance Index and Development - November 24, 2023
- Difference Between Environmental Intervention and Development - November 8, 2023
- Difference Between Eco Efficiency and Eco Effectiveness - September 18, 2023


I like your Explanation
Reply
Clearly explained. Thanks for the article.
Reply
How does activated charcoal find its application in cosmetics?
Reply
Very educative
Reply
Very useful thanks
Reply
You made a mistake in your table. You said that charcoal is used for filtration, and activated carbon is not. It is the other way around, activated carbon is used for filtration. Wouldn’t want someone to try and filter water with charcoal.
Reply