Difference between Sigma and Pi bond
What is Sigma bond?
Sigma bonds are bonds between atoms within molecules formed along the axis connecting the bound nuclei of the atoms.
Molecular bonds
Molecules form when atoms exchange or share electrons through chemical bonding. There are essentially three types of bonds. Ionic bonds, metallic bonds, and covalent bonds. In ionic bonds, the atoms will simply exchange an electron so that one atom will become positively charged and the other negatively charged, causing them to become attracted by the electromagnetic force. In metallic bonds, electrons will be uniformly distributed through the entire molecule creating a sea of free, delocalized electrons enveloping positively charged ions attracted to the electrons.
Within covalent bonds, electrons are shared and the way that they are shared is through the probability clouds of the electrons, and the orbitals in which they are located, overlapping in a manner that is roughly symmetric.
Orbitals and sigma bonds
Orbitals are regions around atoms associated with certain energy levels. Electrons in orbitals farther from the nucleus will have more energy than electrons in orbitals closer to the nucleus. When the orbitals of one atom overlap with the orbitals from another atom, they form molecular orbitals that allow for molecular bonds which, of course, allow for molecules.
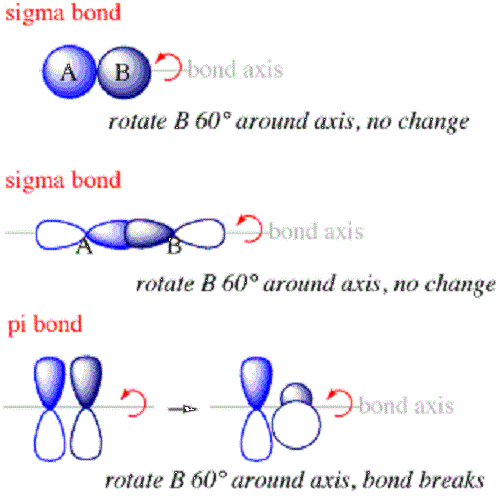
Sigma bonds are the first type of bond that will form between atoms. Within a sigma bond, the electron probability clouds will be along the axis connecting the nuclei of the bonded atoms. Sigma bonds will typically form when s orbitals from different atoms overlap to create a bond. They will always form along the axis between the two nuclei because the s orbital is arranged in something like a sphere around the nucleus.
Sigma bonds and sigma orbitals
The electrons making up the sigma bond will be within the sigma orbitals and thus will be somewhere along the axis connecting the nuclei of the bonded atoms. The sigma bond can, however, be stable or unstable depending on whether the electrons are in a sigma bonding orbital or an anti-bonding orbital.
Sigma bonding orbitals will be in the space between the nuclei whereas anti-bonding orbitals will be along the axis connecting the nuclei but on the sides of the atoms opposite the space between them. The sigma bond will be stable if more electrons are in the bonding orbitals and unstable if more are in the antibonding orbitals or if there is an equal number of electrons in both.
What is Pi bond?
Pi bonds are bonds between atoms within molecules where the electrons are above and below the axis connecting the nuclei of the joined atoms but not along the axis. They are the second type of bond which will form within a molecule after the sigma bond.
Pi bonds and p orbitals
The reason that pi bonds form above and below the bonding axis but not along it is because they form usually from overlapping orbitals such as p orbitals on the bonded atoms. These orbitals do not have an electron density at the nucleus. As a result, the electrons making up the pi bonds that form from the overlapping p orbitals will always cluster in a region that is not directly adjacent to the nucleus. Pi bonds can also form between other atomic orbitals, such as d orbitals which have features in common with p orbitals.
Pi bonds and pi orbitals
When p orbitals of different atoms overlap, they create molecular pi orbitals which allow for pi bonds to form. The bond can once again be stable or unstable depending on the orbital in which the electron is located. The pi bond will be stable if more electrons are in the pi bonding orbitals. It will be unstable if more are in the anti-bonding orbitals or if an equal number are in both.
Similarities between sigma bonds and pi bonds
Sigma bonds and pi bonds are both based on specific molecular orbitals which are derived from the overlapping of particular atomic orbitals, for example, s orbitals in the case of sigma bonds and p orbitals in the case of pi bonds. They also can be stable or unstable depending on whether electrons are in the bonding molecular orbitals or anti-bonding molecular orbitals.
Differences between sigma bonds and pi bonds
Despite their similarities, there are important differences.
- Electrons making up sigma bonds will be distributed in the space along the axis connecting the joined nuclei whereas electrons within pi bonds will be distributed above and below the axis but not along it.
- Sigma bonds are the first bonds to form between atoms within molecules whereas pi bonds are the second.
- Sigma bonds are often formed by the combination of s orbitals in different atoms whereas pi bonds are formed from the combination of p and similar orbitals in different atoms.
- Additionally, the orientation of overlapping orbitals that form pi bonds will be perpendicular to that of overlapping orbitals that form sigma bonds.
Sigma bonds vs. pi bonds
| Sigma bond | Pi bond |
| Atomic orbitals overlap along bonding axis | Atomic orbitals overlap above and below bonding axis |
| First bonds to form between atoms within molecules | Second bonds to form between atoms within molecules |
| Formed from overlapping orbitals such as s orbitals | Formed from overlapping orbitals such as p orbitals |
| Overlapping orbitals perpendicular to those of pi bonds | Overlapping orbitals perpendicular to those of sigma bonds |
Summary: Sigma and Pi Bonds
The sigma bond is a bond between atoms within a molecule which is formed often by s orbitals overlapping along the axis connecting the joined nuclei. It is the first to form and its stability depends on how the electrons are distributed in the sigma bonding and antibonding orbitals. Pi bonds are molecular bonds formed often from overlapping p orbitals from different atoms. The electrons making up pi bonds will be distributed above and below the axis connecting the nuclei of the bonded atoms but not along the axis. The stability of these bonds also depends on the bonding and antibonding pi orbitals. Sigma bonds will be the first bonds to form within molecules while pi bonds will be the second bonds to form. Pi bonds also form from atomic orbitals that are oriented perpendicular to the ones making up sigma bonds.
- Difference Between Environmental Performance Index and Development - November 24, 2023
- Difference Between Environmental Intervention and Development - November 8, 2023
- Difference Between Eco Efficiency and Eco Effectiveness - September 18, 2023
Search DifferenceBetween.net :
Leave a Response
References :
[0]Adamson, Arthur. A textbook of physical chemistry. Elsevier, 2012.
[1]Clayden, J., N. Greeves, and S. Warren. "Organic Chemistry, 2nd." (2012).
[2]Barnes, Craig E. "Inorganic Chemistry (Catherine E. Housecroft and Alan G. Sharpe)." Journal of Chemical Education 80 (2003): 747.
[3]Pauling, Linus. The nature of the chemical bond and the structure of molecules and crystals: an introduction to modern structural chemistry. Vol. 18. Cornell university press, 1960.
[4]Albright, Thomas A., Jeremy K. Burdett, and Myung-Hwan Whangbo. Orbital interactions in chemistry. John Wiley & Sons, 2013.
[5]Streitwieser, Andrew, Clayton H. Heathcock, and Edward M. Kosower. Introduction to Organic Chemistry. New York: Macmillan, 1992. Print.
[6]"Image Credit: https://chemistry.stackexchange.com/questions/68480/why-can-a-sigma-bond-rotate"